At Exelead, we approach contract manufacturing with focused expertise on liposomal and PEGylated formulations to treat rare diseases and small or underserved populations. We specialize in the development and manufacture of lipid-based, parenteral drug products, and our team has more than 20 years' experience working with a wide range of preclinical, clinical and commercial contract manufacturing customers.
What are liposomes, and how are they used in drug delivery?
Liposomes are specialized delivery vehicles that serve multiple roles in enhancing the capabilities of active pharmaceutical ingredients (APIs). First, they can shield a drug from detection by the body’s immune system, mimicking biological membranes and giving the drug more time to reach its intended destination. Second, they serve to help solubilize highly lipophilic drug molecules or modulate the pharmacokinetics and biodistribution of the API—thereby helping to minimize side effects and enhance the product safety profile.
Liposomes possess a unique vesicular structure. These vesicles are composed of a lipid bilayer that forms in the shape of a hollow sphere encompassing an aqueous phase. As such, any cargo of interest can be encapsulated within liposomes in either the aqueous compartment (if it is water-soluble/hydrophilic) or within the lipid bilayer (if fat-soluble/lipophilic).
Some of the primary lipids used to make liposomes are phospholipids and sphingolipids. These two categories of lipids are unique in terms of a head group that is water-loving/hydrophilic and a tail group that is water-hating/lipophilic. Due to their amphiphilic nature, these molecules spontaneously self-assemble to form liposomes and other unique 3D structures when added to aqueous solutions. The shape or morphology of the 3D structures is dependent on a variety of different factors—for example, lipid composition, temperature, pH or the presence of other buffers, salts and sugars in the water.
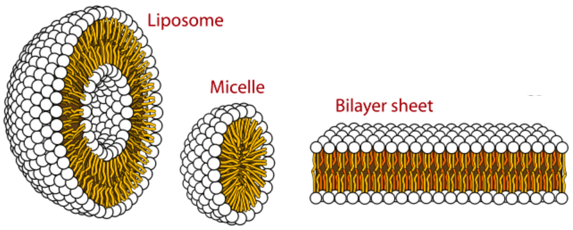
Image Source: Chem Libre Texts
How does a drug interact with the body and become available for use when it is formulated as a liposomal drug product?
In recent years, liposomes have attracted significant attention as a trusted class of drug delivery vehicles. Their self-closed structures can encapsulate multiple drugs at once, protecting enclosed cargo from hydrolysis and breakdown. Additionally, targeting proteins and surface functional ligands on the outer shell of the lipid bilayer can add novel functionality—enabling targeted entry of liposomes into cells, either via antibodies or receptor-targeted ligands. These ligands attach to cell receptors that are over-expressed in certain diseased cells, allowing entry of the drug through the cell membrane.
“Liposomes protect loaded drug molecules from external degradation, and their similarity to biological membranes provides unique opportunities to deliver drug molecules into cells or subcellular compartments ... In addition, various physicochemical properties of liposomes—including their size, charge, and surface functional ligands—can be altered, resulting in functionalities favoring specific drug delivery tasks. These advantages have made liposomes a leading drug delivery platform with a wide range of uses in the clinic.”
- Liposome-like nanostructures for drug delivery | Gao W, Hu C-MJ, Fang RH, Zhang L 1
Typically, liposomes are manufactured as sterile injectables for delivery to the bloodstream, and release of the drug takes place when lipid envelopes break down—which can happen in extracellular or intracellular environments.
Various strategies have been employed to design conventional liposomes with triggered-release capabilities, enhancing therapeutic efficacy by causing the liposomes to release the encapsulated API or “cargo” based on a stimulus response. This release is either driven by conventional breakdown of the liposomes, or it is driven by active stimuli and environmental cues, including thermal energy, pH gradient and shear stress.1
Formulations geared for release in intracellular environments can include pH-sensitive lipids that change the liposomal structure or degrade within acidic compartments, enabling the release of the encapsulated drug. Alternatively, thermosensitive or photosensitive components are sometimes included to enable breakdown and structure modulation due to changes in temperature or reaction to light of certain wavelengths.
Liposome-like drug carriers can come in many different varieties, exhibiting a wide range of biochemical and biophysical properties. While this is advantageous and enables these lipid-based particles to assume useful applications in hundreds of different settings, the number of potential variations makes rigorous manufacturing control imperative.2
Exelead has been manufacturing lipid-based drugs since the early 1990’s, starting with Abelcet. Amphotericin B, the active ingredient in Abelcet, is held within a lipid complex and selectively fuses with fungal membranes to target disease cells.
Liposomes vs. lipid nanoparticles
Liposomes and lipid nanoparticles (LNPs) are similar by design, but slightly different in composition and function. Both are lipid nanoformulations and excellent drug delivery vehicles, transporting cargo of interest within a protective, outer layer of lipids. In application, however, LNPs can take a variety of forms.
LNPs are liposome-like structures especially geared towards encapsulating a broad variety of nucleic acids (RNA and DNA); and as such, they are the most popular non-viral gene delivery system. Exelead develops and manufactures LNPs to encapsulate different types of genetic payloads including siRNA, mRNA and saRNA.
Traditional liposomes include one or more rings of lipid bilayer surrounding an aqueous pocket, but not all LNPs have a contiguous bilayer that would qualify them as lipid vesicles or liposomes. Some LNPs assume a micelle-like structure, encapsulating drug molecules in a non-aqueous core.
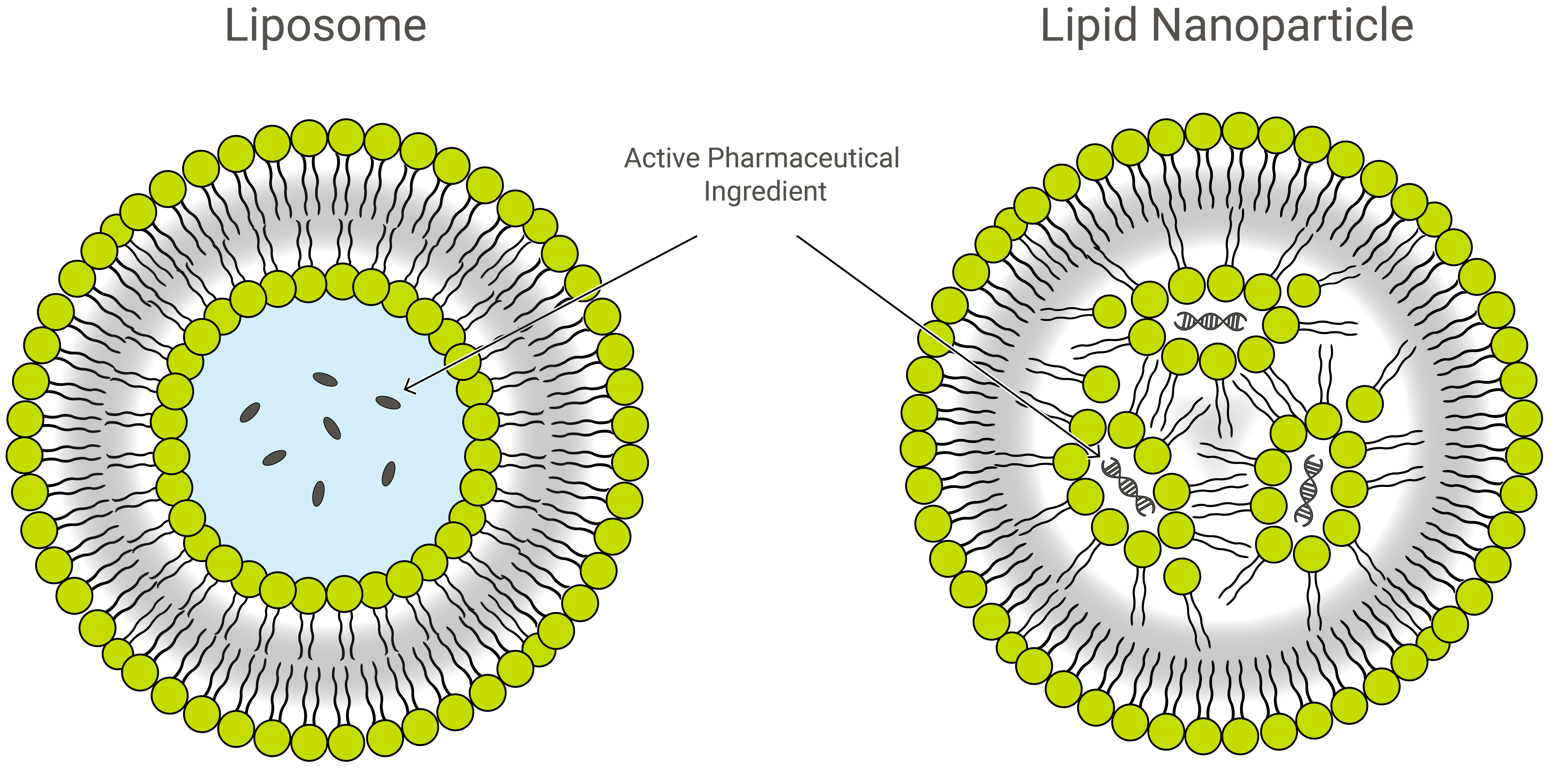
PEGylation of LNPs and liposome-like drug delivery structures
LNPs are composed primarily of cationic lipids (see gene therapy below) along with other lipid ingredients. These typically include neutral phospholipid molecules belonging to the phosphatidylcholine (PC) class and sterols, such as cholesterol. Another common lipid ingredient is what is known as a PEGylated phospholipid—a polyethylene glycol (PEG) polymer covalently attached to the head-group of a phospholipid.
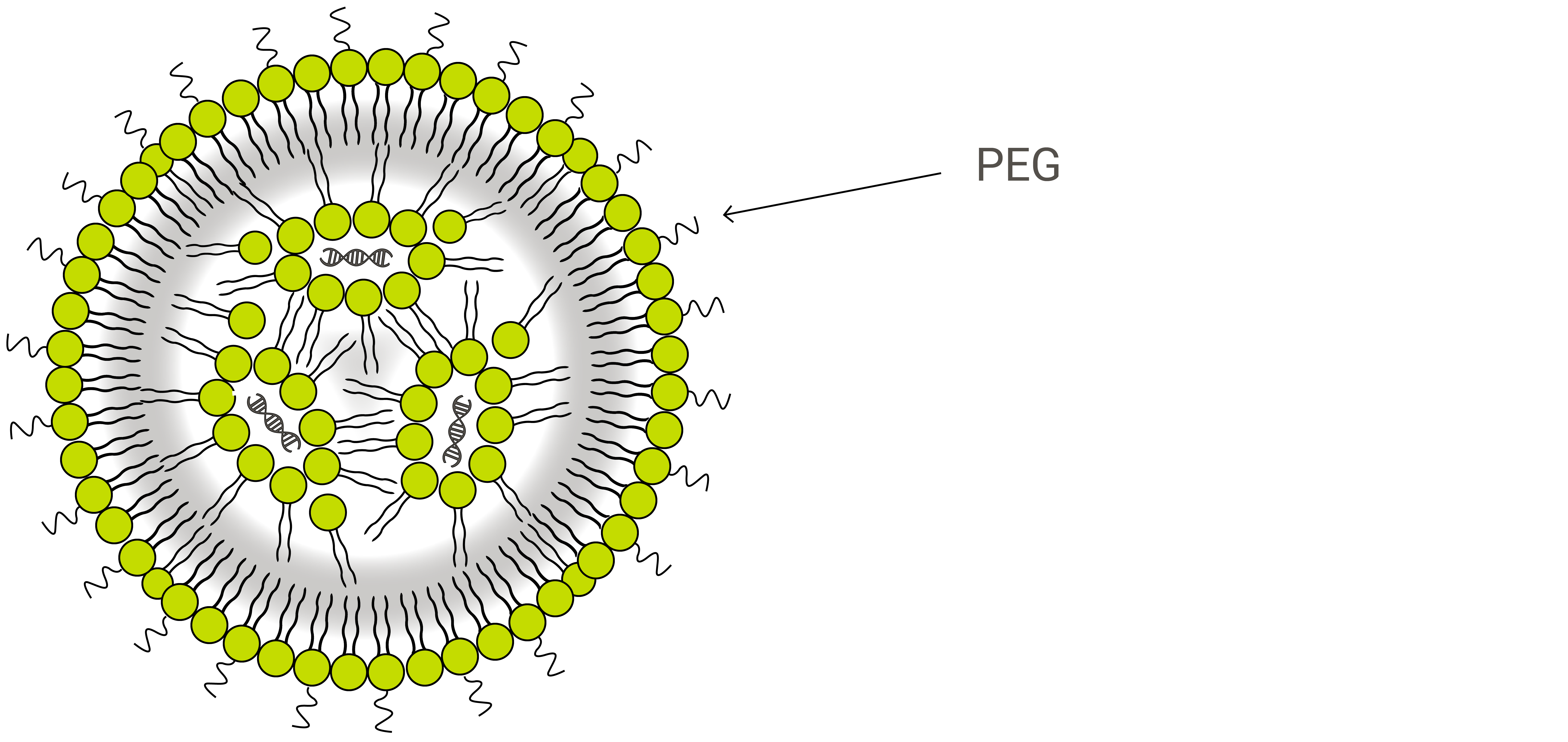
PEGylated phospholipids are used in many lipid-based drug carriers primarily because they offer what is known as a stealth effect to the drug product as it circulates within the body. The human immune system is driven to protect the body from any foreign object, and medicinal nanoparticles are no exception. To aid in delivery efficiency and to allow more circulation time for cargo molecules to reach intended diseases sites, PEG is added to shield these nanoparticles by preventing blood plasma proteins from absorbing into the liposome surface, increasing bloodstream circulation lifetime.1
The second benefit of PEGylation is a boost in stability for liposome-like nanostructures. Conventional liposomes, particularly those smaller than 200 nm in size, can be unstable on their own and tend to fuse with each other to reduce surface tension. This can result in loss of the encapsulated drug or unfavorable mixing of different vesicles’ cargo. One way drug manufacturers have learned to overcome this problem is by covering the exterior of liposomes with polymers like PEG.
These stealth-equipped nanoparticles have resulted in a new generation of liposomal formulations and multiple clinically-approved products. PEGylated liposomes and LNPs are currently the new paradigm for most cancer therapeutics.
LNPs in gene therapy
For a long time, the most effective way to deliver gene-based therapeutics to human cells was to use a virus that had been modified to carry medicinal cargo rather than harmful, self-replicating genes. This method is still occasionally used today, and is referred to as viral gene delivery. Non-viral gene delivery, however, has become popular over the last 20 years due to enhanced safety profiles, lower rates of adverse immunogenic reactions and ease of manufacturing. One of the primary drivers of this movement has been the development of lipid and polymer-based carriers, of which LNPs are the most popular.
LNPs used to deliver genes are primarily synthesized using cationic, or positively-charged, lipids that associate with anionic, or negatively-charged, nucleic acids. Other lipid-based components can also be added to modulate the delivery efficiency and location release of the genetic cargo. LNPs also provide mechanical stability, controlled morphology and narrow size distribution.1
Inorganic materials, organic materials and hydrogels have each been explored as cores for liposomal nanoparticles, encapsulated within varying numbers of lipid layers that form the shell. One of the most successful variations of these hybrid nanoparticles incorporates PLA or PLGA polymers within a lipid monolayer. These two core biopolymers are particularly useful in drug delivery because they facilitate controlled drug release.1
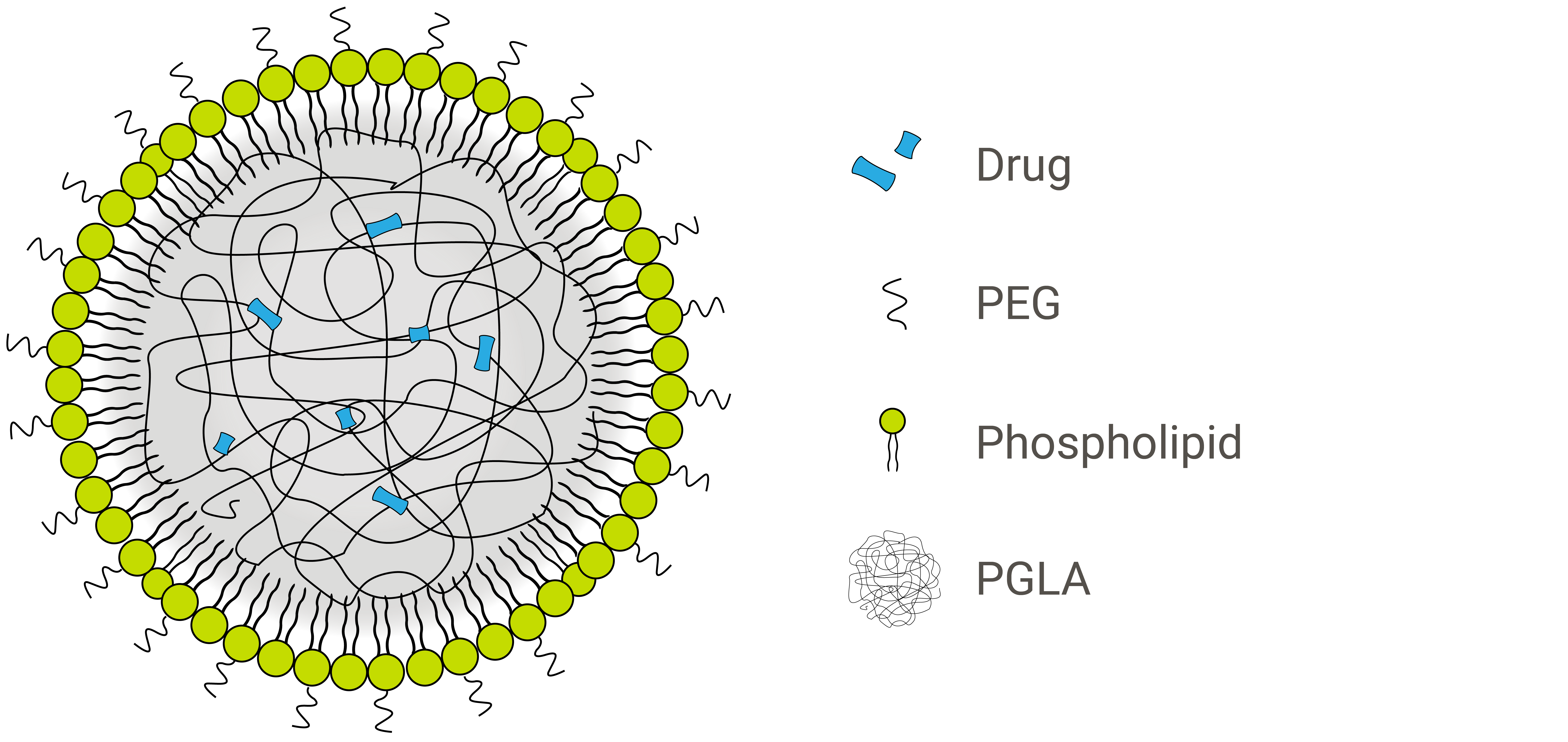
The nanoparticle formulation shown above is a lipid-polymer hybrid with a hydrophobic PLGA core and a hydrophilic lipid-PEG shell.
An expanding field
“Liposomes have come a long way to become a class of validated drug carriers … An increasing variety of liposome-like nanostructures are under development, each with unique strengths suitable for specific drug delivery tasks. Meanwhile, understanding [of] the interactions between these nanostructures and biological systems is rapidly progressing. A substantial amount of information on their circulation time, tissue accumulation, and potential toxicity has been obtained. It is certain that liposome-like nanocarriers will play a larger role for drug delivery in the foreseeable future.”
- Liposome-like nanostructures for drug delivery | Gao W, Hu C-MJ, Fang RH, Zhang L 1
While there is significant work ongoing in the development of controlled-release, nano-compartmentalized medicinal agents, liposomes and LNPs are especially promising options. These structures provide a unique, naturally stable, cell-like morphology for nanomedicines, and are poised to progress towards more advanced therapeutic strategies. Exelead is taking on such challenges, including the development of nanogels that incorporate an array of biologics and small molecules.
Since liposomes were first proposed as a drug delivery system in the late 1960s, variations in structure and functionality have emerged, providing valuable advancements in terms of disease targeting. LNP drugs have cropped up across the pharmaceutical industry as therapies designed to deliver anti-cancer agents, antibiotics, gene medicines, anesthetics and anti-inflammatory drugs.3
“In clinical applications, liposomal drugs have been proven to be most useful for their ability to ‘passively’ accumulate at sites of increased vasculature permeability, when their average diameter is in the ultrafilterable range (<200 nm in diameter), and for their ability to reduce the side effects of the encapsulated drugs relative to free drugs. This has resulted in an overall increase in therapeutic index, which measures efficacy over toxicity.”
- Liposomal drug delivery systems: from concept to clinical applications | Allen TM, Cullis PR 3
Applications in personalized medicine—a new era in therapeutic strategies
In contrast with traditional, big-pharma approaches to treatment of disease, personalized medicine takes into account individual differences in lifestyle, environment, and biology—including a patient’s genetics.
This is extremely applicable for diseases like cancer. Even within a single type of cancer, tumor types differ from one patient to another, and understanding the particular genetic mutation a patient has developed allows doctors to employ more specific and precise treatments.4
With the advent of personalized genetic therapies, doctors and scientists can effectively tailor an active pharmaceutical ingredient—often RNA or DNA—to match the specific disease profile of a particular patient or small group of patients. This approach to hyper-specific disease targeting increases efficacy and decreases unwanted side effects for groups of similar patients.
LNPs as delivery vehicles for oligonucleotides
Because so much of the growing field of personalized medicine is focused on genetic therapies, LNPs have become particularly useful as a drug delivery platform. Any oligonucleotide could theoretically be encapsulated within a liposome or LNP, but siRNA are currently the most common cargo in these types of drug products.
In theory, segments of siRNA can be designed to silence any gene, which is an exciting concept for both doctors and researchers. Unfortunately, delivery of free, unencapsulated RNA into human cells is difficult, as they are large, unstable in serum and prone to nuclease degradation.5
While researchers have made attempts to stabilize siRNA in serum by adding phosphorothioate linkages, high doses are required to effectively silence genes in humans. LNPs have provided a solution to this problem by providing flexible and easy means of encapsulation, protecting the siRNA segments until they reach their intended destination and facilitating their delivery into target cells.5
“LNPs containing ionizable cationic lipids have a number of features necessary for the systemic delivery of polynucleic acids, including small sizes, serum stability, low surface zeta potentials at physiological pH, and cationic charge at acidic pH values (e.g., in endosomes). Further, by taking advantage of ‘endogenous’ targeting processes due to association with ApoE following administration, highly efficient uptake into hepatocytes can be achieved following i.v. administration, leading to excellent gene silencing capabilities.”
- Lipid nanoparticle delivery systems for siRNA-based therapeutics | Wan C, Allen TM, Cullis PR 5
Smaller batch sizes
Personalized drug products are often manufactured in small batch sizes for single patients or small populations and can frequently result in less than one liter of product. In contrast, traditional manufacturing batches for mainstream pharmaceuticals often produce thousands of liters of drug product at scale. Personalized medicine requires a unique approach, and each batch must be manufactured under stringent cGMP conditions.
As personalized medicine has become a prominent focus in drug development, many companies in the pharmaceutical manufacturing industry have adapted their pipelines to accommodate smaller batches slated for small groups of patients in addition to traditional, large-scale drug production.
At Exelead, extensive efforts have been made to accommodate these small-batch therapeutics, which often require expensive API and quick turnaround time. Patient or antigen-specific drugs don’t leave much time from the moment the oligonucleotide is sequenced to the time the product needs to be formulated at our site. These short-term forecasts, sometimes only six weeks, present challenges that we have been able to overcome by refining our existing systems and incorporating innovative formulation techniques.
Widespread applications
While personalized medicine has the potential to treat almost any disease, current research has primarily focused on 1) immunotherapies, 2) conventional therapies augmented via pharmacogenomics and 3) biomarker-related cancer treatments. Liposomes and LNPs have application as delivery vehicles for each of these categories of drug products, making them an indispensable asset in this new field of pharmaceutical development.

Reference Articles
- Gao W, Hu C-MJ, Fang RH, Zhang L. Liposome-like Nanostructures for Drug Delivery. Journal of materials chemistry B, Materials for biology and medicine. 2013;1(48):10.1039/C3TB21238F. doi:10.1039/C3TB21238F. [NCBI]
- Kraft JC, Freeling JP, Wang Z, Ho RJY. Emerging Research and Clinical Development Trends of Liposome and Lipid Nanoparticle Drug Delivery Systems. Journal of pharmaceutical sciences. 2014;103(1):29-52. doi:10.1002/jps.23773. [NCBI]
- Theresa M. Allen, Pieter R. Cullis. Liposomal drug delivery systems: From concept to clinical applications. Advanced Drug Delivery Reviews. 2013 Jan;65(1):36–48
- Esposito L. What Does Personalized Medicine Really Mean? US News: Health Care. 2018 Jan; [US News]
- Wan C, Allen TM, Cullis PR. Lipid nanoparticle delivery systems for siRNA-based therapeutics. Drug Deliv Transl Res. 2014 Feb;4(1):74-83. doi: 10.1007/s13346-013-0161-z.
- Vogenberg FR, Isaacson Barash C, Pursel M. Personalized Medicine: Part 1: Evolution and Development into Theranostics. Pharmacy and Therapeutics. 2010;35(10):560-576. [NCBI]
[/col] [col-1-6 data-mobile="hidden"] [/col] [/grid]